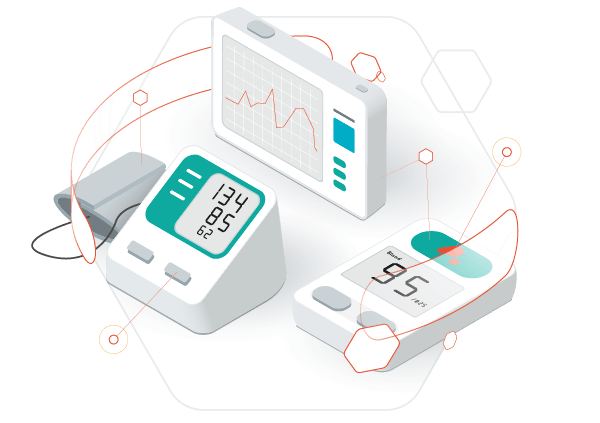
Take advantage of proven and efficient software technologies in order to create safe, robust and reliable medical devices than will not only impress your end customer, but also ensure product quality and patient safety.
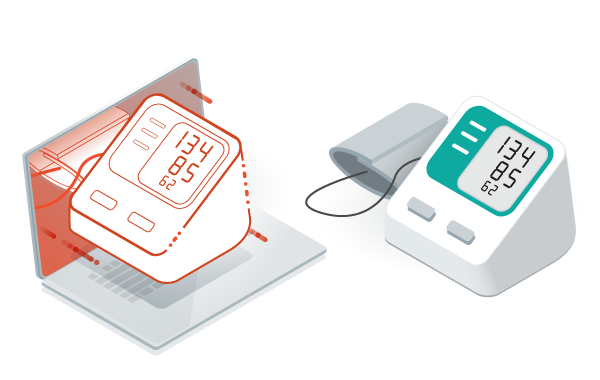
The MICROEJ VEE uses the same virtualization principles that are heavily used in computer desktops for over 20 years, only on a much smaller footprint.
This achievement enables medical devices manufacturers to add some agility into their software development process by taking advantage of object oriented programming, simulation with virtual devices, and automated testing for a faster and more flexible software development (for example: user interface design) that conforms to the latest compliance requirements.
MicroEJ solutions drastically reduce software complexity and simplify certification efforts for compliance to IEC 62304 class A and B, CLSI LIS01-A2, CLSI LIS02-A2 and other major and emerging medical market’s certification and compliance efforts.